EPIDIOLEX® (cannabidiol) offers flexible dosing for tolerability and response optimization
Recommended dosing and titration
Increase in weekly increments of 5 mg/kg/day (2.5 mg/kg twice daily).*
Week 1
(2.5 mg/kg twice daily)
Week 2
(5 mg/kg twice daily)
Week 3
(7.5 mg/kg twice daily)
Week 4
(10 mg/kg twice daily)
Week 5
(12.5 mg/kg twice daily)


Dose adjustment and a slower dose titration are recommended in patients with moderate or severe hepatic impairment. Select an option above to view dosing recommendations for patients with hepatic impairment.
*For patients in whom a more rapid titration is warranted, the dosage may be increased no more frequently than every other day.
†For patients with LGS or Dravet syndrome, administration of the 20-mg/kg/day dosage resulted in somewhat greater reductions in seizure rates than the recommended maintenance dosage of 10 mg/kg/day, but with an increase in adverse reactions. Increase dose from 10 mg/kg/day if tolerated and required.
‡The effectiveness of doses lower than 25 mg/kg/day has not been studied in patients with TSC.
Because of an increase in exposure to EPIDIOLEX, dose adjustment and slower titration are recommended in patients with moderate or severe hepatic impairment. Consider not initiating EPIDIOLEX in patients with evidence of significant liver injury. Dose adjustments are not required in patients with mild hepatic impairment.
Review additional monitoring considerations
Example dosing and titration — 1/2 standard dosing*
Week 1
(1.25 mg/kg twice daily)
Week 2
(2.5 mg/kg twice daily)
Week 3
(3.75 mg/kg twice daily)
Week 4
(5 mg/kg twice daily)
Week 5
(6.25 mg/kg twice daily)


*Note: Total dosage should be split and administered twice daily. See additional dosing considerations for more information.
Because of an increase in exposure to EPIDIOLEX, dose adjustment and slower titration are recommended in patients with moderate or severe hepatic impairment. Consider not initiating EPIDIOLEX in patients with evidence of significant liver injury. Dose adjustments are not required in patients with mild hepatic impairment.
Review additional monitoring considerations
Example dosing and titration — 1/5 standard dosing*
Week 1
(0.5 mg/kg twice daily)
Week 2
(1 mg/kg twice daily)
Week 3
(1.5 mg/kg twice daily)
Week 4
(2 mg/kg twice daily)
Week 5
(2.5 mg/kg twice daily)


*Note: Total dosage should be split and administered twice daily. See additional dosing considerations for more information.
Dosing considerations

Food may affect EPIDIOLEX levels
EPIDIOLEX is an oral solution that is compatible with a ketogenic diet. EPIDIOLEX should be administered consistently with respect to meals. If necessary, EPIDIOLEX can be administered with certain nasogastric tubes (NG-tube) or gastrostomy tubes (G-tube). For specific instructions, please see the EPIDIOLEX Prescribing Information.

EPIDIOLEX can cause dose-related elevations of liver transaminases
Because of the risk of hepatocellular injury, obtain serum transaminases (ALT and AST) and total bilirubin levels in all patients prior to starting treatment with EPIDIOLEX and periodically thereafter.
- Because of an increase in exposure to EPIDIOLEX, dose adjustment and slower dose titration are recommended in patients with moderate or severe hepatic impairment. Consider not initiating EPIDIOLEX in patients with evidence of significant liver injury. Dose adjustments are not required in patients with mild hepatic impairment

Taking EPIDIOLEX with other drugs
Strong inducers of CYP3A4 and CYP2C19 may affect EPIDIOLEX exposure. EPIDIOLEX may affect exposure to CYP2C19 substrates (eg, clobazam, diazepam, stiripentol), orally administered P-gp substrates, or other substrates (see full Prescribing Information). Consider dose reduction of orally administered everolimus, with appropriate therapeutic drug monitoring, when everolimus is combined with EPIDIOLEX. A lower starting dose of everolimus is recommended when added to EPIDIOLEX therapy. Concomitant use of EPIDIOLEX and valproate increases the incidence of liver enzyme elevations. Pneumonia was observed more frequently with concomitant use of EPIDIOLEX and clobazam. Dosage adjustment of EPIDIOLEX or other concomitant medications may be necessary.
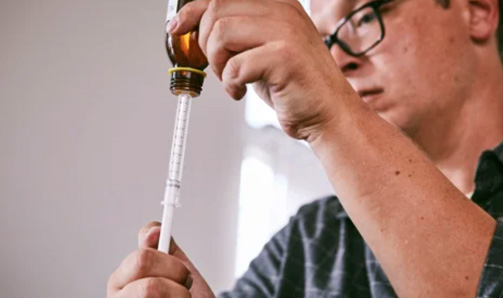
Administering EPIDIOLEX
Step-by-step instructions for administration.
Writing a prescription
Sample prescription
To help avoid delays obtaining coverage for patients when prescribing, provide the:
- Dosing/titration schedule
- Patient weight
- ICD-10 diagnosis code

ICD-10 diagnosis codes
LGS
- G40.811
- G40.812
- G40.813
- G40.814
Dravet syndrome
- G40.833
- G40.834
TSC
- Q85.1
This is provided for example purposes only. Not intended to supersede independent clinical judgment or institutional protocols. Please see full Prescribing Information for recommendations on liver monitoring.
As certain health plans and institutions may have policies regarding cannabinoid products/use, prescriptions should include EPIDIOLEX by name to avoid rejection.
If dose adjustment is required mid-prescription cycle, a new prescription will be needed.