CONVULSIVE SEIZURE REDUCTION
EPIDIOLEX®
(cannabidiol)
significantly reduced convulsive seizures in patients living with Dravet syndrome
Reduction in monthly frequency of convulsive seizures
Results from the 14-week treatment period. Convulsive seizures included all countable atonic, tonic, clonic, and tonic-clonic seizures.
Patients at baseline1:
- Had previously tried a median of 4 prior ASMs
- Currently uncontrolled with a median of 3 current ASMs
In Study 3, 93% of patients were taking ≥2 ASMs at baseline and still experiencing a median of 13 convulsive seizures per 28 days.1
The most commonly used concomitant ASMs were:
65% clobazam |
57% valproate |
43% stiripentol
Seizure frequency reduction was reported as early as Day 10 in a post hoc analysis of the Dravet syndrome clinical trial.2
Recommended daily dosage is 10 mg/kg/day (5 mg/kg twice daily), with a maximum maintenance dosage of 20 mg/kg/day (10 mg/kg twice daily).
Administration of the 20 mg/kg/day dosage resulted in somewhat greater reductions in seizure rates than the recommended maintenance dosage of 10 mg/kg/day, but with an increase in adverse reactions. Patients with moderate to severe hepatic impairment require a dose adjustment.
RESPONDER RATES
EPIDIOLEX®
(cannabidiol)
cut seizure frequency by ≥50% and ≥75% in more patients than placebo in the Dravet syndrome trial
Responder rates (≥50% and ≥75% reductions in convulsive seizures from baseline)1
Results from the 14-week treatment period.
More patients achieved freedom from convulsive seizures with EPIDIOLEX than with placebo.
7%
EPIDIOLEX
20 mg/kg/day
0%
PLACEBO
3-YEAR OPEN-LABEL EXTENSION
3-year sustained reduction of convulsive seizures3
Open-label extension: Reduction in monthly frequency of convulsive seizures3
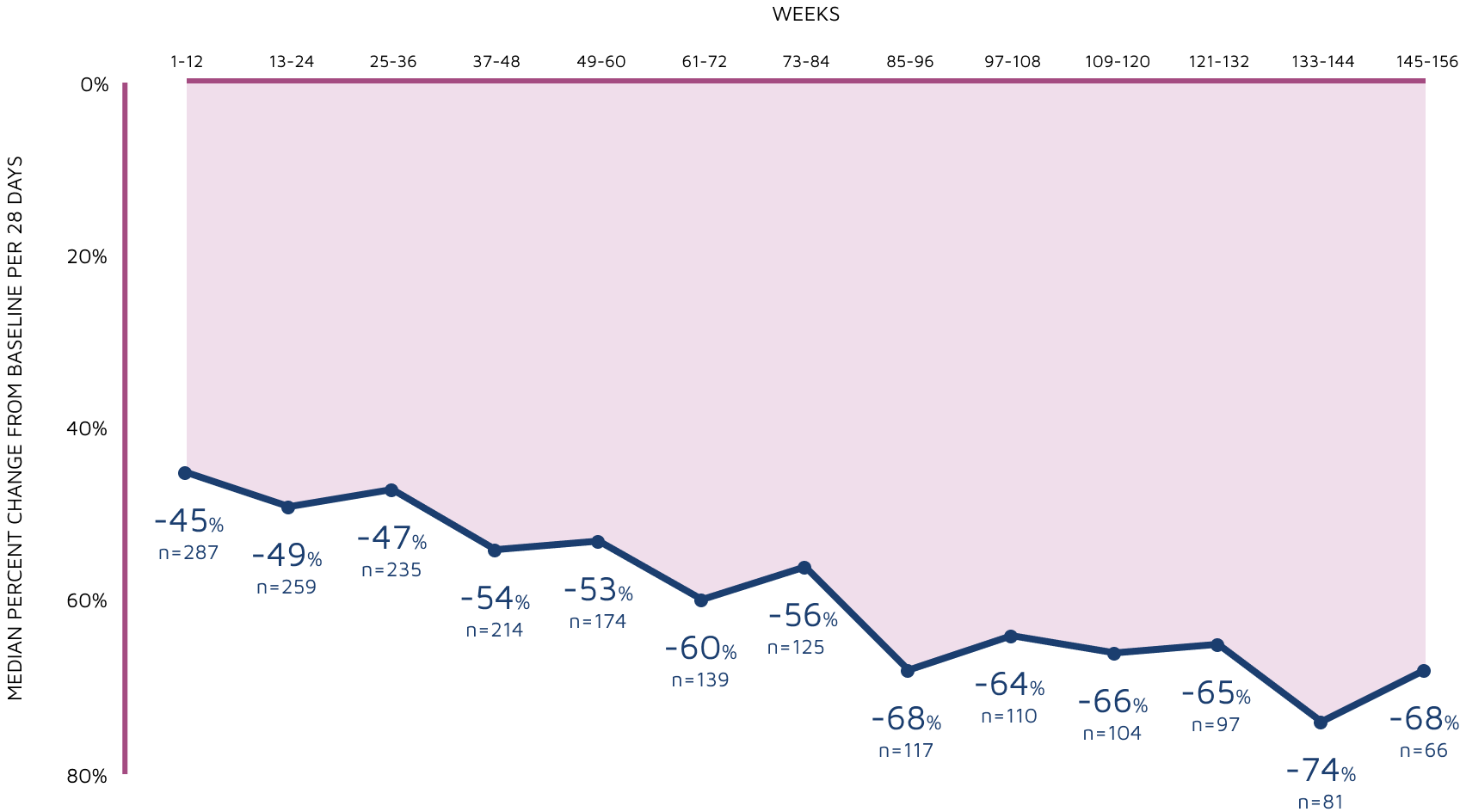
WEEKS

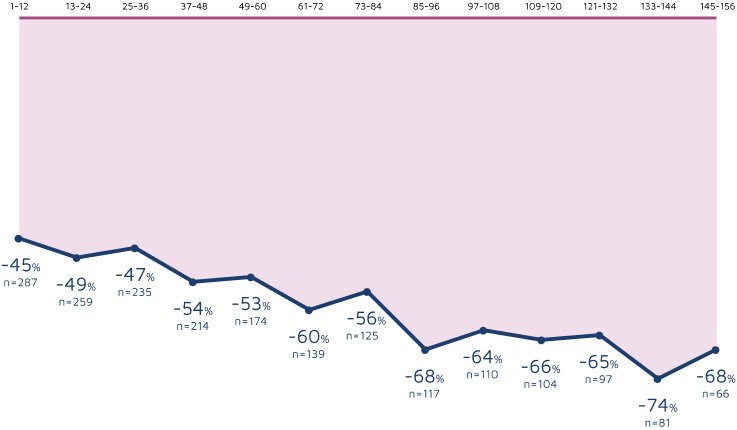
Decreasing n-values reflect a combination of discontinuations and rolling entry into the open-label extension trial.3
- Retention rates at 1, 2, and 3 years were 72%, 53%, and 45%, respectively3
- 8% (n=25) of withdrawals were due to adverse reactions3
- LOCF sensitivity analyses were carried out on change in seizure frequency data to assess the effects due to withdrawal of patients3
Adverse events:
- The long-term safety profile of EPIDIOLEX®
(cannabidiol)in this open-label extension trial was generally similar to that observed in the EPIDIOLEX clinical development program3
- Five deaths were reported in patients with Dravet syndrome, all of which were deemed unrelated to treatment by the investigator3
- In the open-label extension trial, titration to doses over 20 mg/kg/day was permitted. At higher doses, an increase in adverse reactions was observed3
LEARN MORE
Additional resources

Diagnosing an Adult Patient With Dravet Syndrome
A case study of a hypothetical patient with Dravet syndrome who has been transferred to your care.
Watch now

Patient characteristics
EPIDIOLEX was studied in a wide range of patients living with LGS, Dravet syndrome and TSC, and with concomitant therapies.
See the data

Safety
The safety profile of EPIDIOLEX was evaluated in an expansive clinical trial program.
See the data