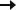EPIDIOLEX® (cannabidiol) is proven across a wide range of patients living with LGS, Dravet syndrome, and TSC, and with concomitant therapies
EPIDIOLEX has a breadth of data in patients living with LGS, Dravet syndrome, and TSC
Patients at baseline1-4
| Median number of seizures at baseline/28 days | Median number of previous ASMs | Median number of current ASMs | Patients taking ≥2 ASMs (%) | |
| LGS Study 1 (ages 2-55 years)1* |
74 (drop seizures) |
6 | 3 | 94% |
| LGS Study 2 (ages 2-55 years)2* |
85 (drop seizures) |
6 | 3 | 94% |
| Dravet syndrome Study 3 (ages 2-18 years)3† |
13 (convulsive seizures) |
4 | 3 | 93% |
| TSC Study 4 (ages 1-65 years)4‡ |
57 (TSC-associated seizures) |
4 | 3 | 89% |
| LGS Study 1 (ages 2-55 years)1* | |
| Median number of seizures at baseline/28 days | 74 (drop seizures) |
| Median number of previous ASMs | 6 |
| Median number of current ASMs | 3 |
| Patients taking ≥2 ASMs (%) | 94% |
| LGS Study 2 (ages 2-55 years)2* | |
| Median number of seizures at baseline/28 days | 85 (drop seizures) |
| Median number of previous ASMs | 6 |
| Median number of current ASMs | 3 |
| Patients taking ≥2 ASMs (%) | 94% |
* Eligibility criteria for age was 2-55 years. 32% of patients in LGS studies were ages 18-55. Both trials had a 4-week baseline period, during which patients were required to have a minimum of 8 drop seizures while on stable ASM therapy.1,2
| Dravet syndrome Study 3 (ages 2-18 years)3* | |
| Median number of seizures at baseline/28 days | 13 (convulsive seizures) |
| Median number of previous ASMs | 4 |
| Median number of current ASMs | 3 |
| Patients taking ≥2 ASMs (%) | 93% |
* In the Dravet syndrome study, eligibility criteria for age was 2-18 years. During the 4-week baseline period, patients were required to have at least 4 convulsive seizures while on stable ASM therapy.
| TSC Study 4 (ages 1-65 years)4* | |
| Median number of seizures at baseline/28 days | 57 (TSC-associated seizures) |
| Median number of previous ASMs | 4 |
| Median number of current ASMs | 3 |
| Patients taking ≥2 ASMs (%) | 89% |
* Eligibility criteria for age was 1-65 years. During the 4-week baseline period, patients were required to have a minimum of 8 TSC-associated seizures (countable focal or generalized), with at least 1 seizure per week in ≥3 of the 4 weeks while on stable ASM therapy.5
* Eligibility criteria for age was 2-55 years. 32% of patients in LGS studies were ages 18-55. Both trials had a 4-week baseline period, during which patients were required to have a minimum of 8 drop seizures while on stable ASM therapy.1,2
† In the Dravet syndrome study, eligibility criteria for age was 2-18 years. During the 4-week baseline period, patients were required to have at least 4 convulsive seizures while on stable ASM therapy.
‡ Eligibility criteria for age was 1-65 years. During the 4-week baseline period, patients were required to have a minimum of 8 TSC-associated seizures (countable focal or generalized), with at least 1 seizure per week in ≥3 of the 4 weeks while on stable ASM therapy.5
EPIDIOLEX delivers broad-spectrum efficacy to significantly reduce generalized (LGS, Dravet syndrome, and TSC) and focal (TSC) seizures
PRIMARY ENDPOINT: CHANGE (PER 28 DAYS) FROM BASELINE VS PLACEBO WHEN ADDED TO CURRENT TREATMENT
LGS |
Dravet syndrome |
TSC |
|
| PROVEN REDUCTIONS IN: | ✔ Drop/total seizures | ✔ Convulsive seizures | ✔ TSC-associated seizures including partial-onset§ and generalized |
| PRIMARY ENDPOINT: |
Drop seizures defined as seizures that led to or could have led to a fall or injury1,2:
|
Convulsive seizures defined as countable3:
|
TSC-associated seizures defined as4:
|
LGS
Drop seizures defined as seizures that led to or could have led to a fall or injury1,2:
- Atonic
- Tonic
- Tonic-clonic
Dravet syndrome
Convulsive seizures defined as countable3:
- Atonic
- Tonic
- Tonic-clonic
- Clonic
TSC
TSC-associated seizures defined as4:
- Partial-onset seizures†
- Atonic
- Tonic
- Tonic-clonic
- Clonic
§†Partial-onset seizures (focal) included simple partial seizures (focal motor seizure), complex partial seizures (focal impaired), and secondary generalized tonic-clonic seizures (focal to bilateral tonic-clonic).6
EPIDIOLEX was studied in 900 patients, the largest clinical trial program to date in LGS, Dravet syndrome, and TSC∥‡
4 RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED, MULTICENTER PHASE 3 TRIALS1-4


∥‡There was a second randomized controlled trial in 199 patients with Dravet syndrome that has been completed since its original approval.7
¶§Patients who completed the randomized controlled trials were eligible to continue in an open-label extension.1,2
Additional resources

LGS data
EPIDIOLEX significantly reduced drop/total seizure frequency in patients living with LGS.
See the data

Dravet syndrome data
EPIDIOLEX significantly reduced convulsive seizures in patients living with Dravet syndrome.
See the data

TSC data
EPIDIOLEX reduced the frequency of TSC-associated seizures.
See the data